Market Overview:
The paroxysmal nocturnal hemoglobinuria market reached a value of US$ 2.9 Billion in 2023 and expected to reach US$ 6.9 Billion by 2034, exhibiting a growth rate (CAGR) of 8.29% during 2024-2034. The paroxysmal nocturnal hemoglobinuria market report offers a comprehensive analysis of the market in the United States, EU5 (including Germany, Spain, Italy, France, and the United Kingdom), and Japan. It covers aspects such as treatment methods, drugs available in the market, drugs in development, the market share of various therapies, and the market’s performance in the seven major regions. Additionally, the report evaluates the performance of leading companies and their pharmaceutical products. Current and projected patient numbers across these key markets are also detailed in the report. This study is essential for manufacturers, investors, business planners, researchers, consultants, and anyone interested or involved in the paroxysmal nocturnal hemoglobinuria market.
Request for a sample of this Report: https://www.imarcgroup.com/paroxysmal-nocturnal-hemoglobinuria-market/requestsample
Paroxysmal Nocturnal Hemoglobinuria Market Trends:
- Overview of PNH:
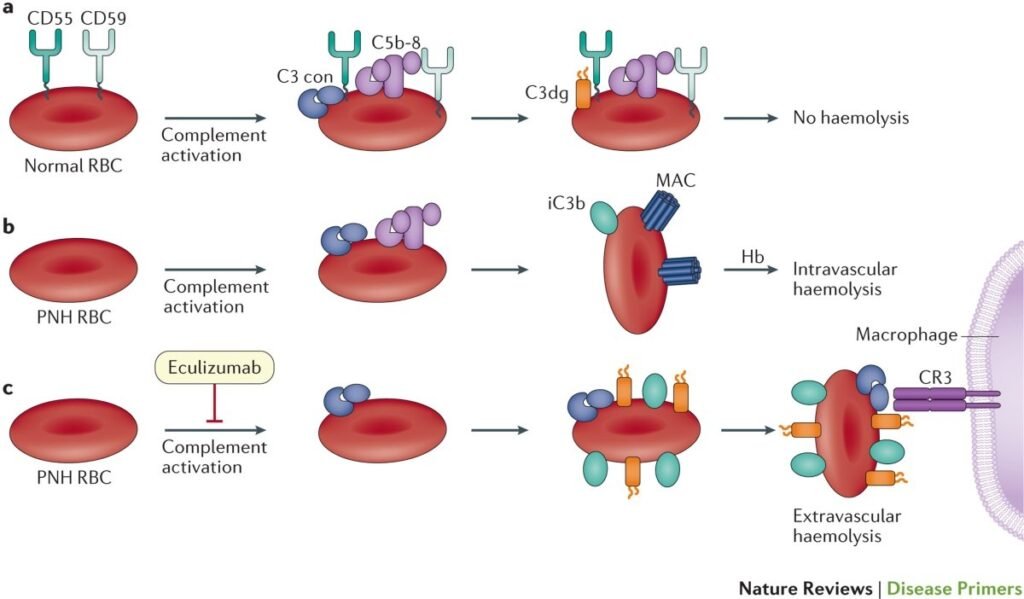
- Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired hematological disorder.
- Characterized by abnormal red blood cell destruction (hemolysis) and the presence of hemoglobin in the urine.
- Market Expansion Drivers:
- Growing prevalence of PNH.
- Improved diagnostic tools such as flow cytometry for early and precise detection.
- Increasing awareness of the condition leading to higher diagnosis and treatment rates.
- Advancements in Treatment:
- Adoption of targeted therapies, particularly complement inhibitors like eculizumab and ravulizumab, enhancing patient outcomes.
- Rise of biosimilars and next-generation complement inhibitors, offering cost-effective and innovative solutions.
- Investment in Research and Development:
- Increased funding by governments and healthcare organizations for PNH research.
- Regulatory incentives such as orphan drug status to encourage the development of novel therapies.
- Role of Digital Health Technologies:
- Integration of telemedicine platforms improving disease monitoring and patient engagement.
- Better treatment adherence due to timely interventions.
- Collaborations and Personalized Therapies:
- Partnerships between biopharmaceutical companies and academic institutions accelerating therapy development.
- Focus on personalized treatments tailored to specific patient needs.
- Future Market Outlook:
- Exploration of gene therapies and curative approaches targeting the root causes of PNH.
- Expected continued growth of the PNH market driven by these innovations.
Countries Covered:
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country:
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the paroxysmal nocturnal hemoglobinuria market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the paroxysmal nocturnal hemoglobinuria market
- Reimbursement scenario in the market
- In-market and pipeline drugs
This report also provides a detailed analysis of the current paroxysmal nocturnal hemoglobinuria market drugs and late-stage pipeline drugs.
In-Market Drugs:
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs:
- Drug overview
- Mechanism of action
- Regulatory status
- Clinical trial results
- Drug uptake and market performance
Competitive Landscape with key players:
The competitive landscape of the paroxysmal nocturnal hemoglobinuria market has been studied in the report with the detailed profiles of the key players operating in the market.
Some of these Key Players:
- Apellis Pharmaceuticals
- Swedish Orphan Biovitrum
- Alexion AstraZencea Rare Disease
- Chugai Pharmaceutical
- Achillion Pharmaceuticals
- Novartis Pharmaceuticals
- Regeneron Pharmaceuticals
- NovelMed Therapeutics
Ask Analyst for Customization and Explore Full Report with TOC & List of Figures: https://www.imarcgroup.com/request?type=report&id=7822&flag=C
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145